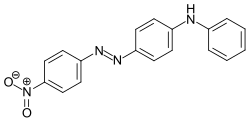
Disperse Orange 1
Chemical compound
 | |
| Names | |
|---|---|
| Other names 4-Anilino-4'-nitroazobenzene C.I. 11080 (Colour index numbers) | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider |
|
| ECHA InfoCard | 100.018.141 |
| EC Number |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C18H14N4O2 |
| Molar mass | 318.33476 g/mol |
| Melting point | 160.0 °C (320.0 °F; 433.1 K) |
| Hazards | |
| GHS labelling: | |
Pictograms |  |
| Warning | |
Hazard statements | H317 |
Precautionary statements | P261, P272, P280, P302+P352, P321, P333+P313, P363, P501 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).  Y verify (what is Y verify (what is  Y Y N ?) N ?) Infobox references | |
Chemical compound
Disperse Orange 1, or 4-anilino-4'-nitroazobenzene, is an azo dye. Commercial samples contain approximately 25% dye by weight, with the remaining mass consisting of NaCl and other salts.
This dye is useful in conducting experiments with flash photolysis due to the isomerization effect between the trans-4A4N and cis-4A4N states that occurs during photo relaxation.[1][2]
References
- ^ Hair, S. R.; Taylor, G. A.; Schultz, L. W. J. (1990). "An Easily Implemented Flash Photolysis Experiment for the Physical Chemistry Laboratory: the Isomerization of 4-Anilino-4'-Nitroazobenzene". Journal of Chemical Education. 67 (8): 709. Bibcode:1990JChEd..67..709H. doi:10.1021/ed067p709.
- ^ Wildes, P. D.; Pacifici, J. G.; Irick, G.; Whitten, D. G. J. Am. Chem. Soc., 1971, 93, 2004.











